每日外闻92
Risk factor for Alzheimer’s disease breaks the blood–brain barrier
阿尔茨海默症的危险因子打破了血脑屏障
作者清晰的回顾了相关问题的研究,主要的结论为下面这张图所示的那样:
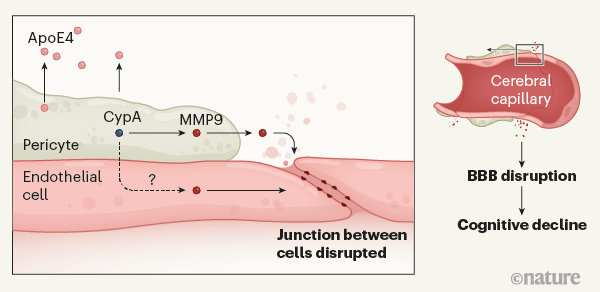
携带APOE4突变的的人罹患阿尔茨海默氏病的风险更高。 Montagne等人提供的证据表明,ApoE4蛋白被称为周细胞(pericytes)的细胞分泌,该细胞与位于血脑屏障(BBB)处脑毛细血管的内皮细胞(endothelial cells)邻接。分泌的ApoE4激活周细胞中的亲环蛋白A(CypA)。这触发了下游信号传导途径,该信号传导途径涉及周细胞中以及内皮细胞中炎性蛋白基质金属蛋白酶9(MMP9)的激活。这引起相邻内皮细胞之间连接的破坏,从而在参与学习和记忆的大脑区域打开了血脑屏障。 BBB的破坏与认知能力受损有关,尽管将两者联系起来的机制尚不清楚。
以下为原文:
-----
Proteins from blood plasma have been found in the cerebrospinal fluid (the liquid that surrounds the brain and spinal cord) of cognitively healthy people who carry APOE4 and who subsequently go on to develop Alzheimer’s disease. These proteins have presumably leaked through the BBB, indicating that the integrity of the barrier is lost before cognition declines5. Evidence from mouse models, and from the brains of people who have died with Alzheimer’s disease, suggests that BBB breakdown is caused by the degeneration of pericytes — cells nestled in the wall of cerebral capillaries. These cells normally safeguard the BBB5 by preventing the breakdown of junctions between endothelial cells, which make up the capillary walls.
Whether ApoE4 is responsible for early BBB dysfunction in Alzheimer’s disease, by itself or in concert with Aβ and tau, was unknown. Montagne and colleagues set out to address this knowledge gap. The authors used a technique called dynamic contrast-enhanced magnetic resonance imaging to investigate the permeability of the BBB in people who had either healthy cognition or mild cognitive impairment (a prelude to Alzheimer’s disease), grouped according to their APOE status. They found that people who were cognitively healthy and carried either one or two copies of APOE4 had a leaky BBB in two brain regions important for memory and cognition — the hippocampus and the parahippocampal gyrus. This leakage was worse in APOE4 carriers who exhibited mild cognitive decline.
Remarkably, these effects preceded any signs of tissue loss in the hippocampus and parahippocampal gyrus, attesting to the idea that BBB disruption is an early event in the onset of neurodegeneration. BBB leakage was independent of Aβ and tau accumulation, which the authors assessed both by studying samples of cerebrospinal fluid and through another brain**-**imaging technique, positron emission tomography. Montagne and co-workers found that, unlike in APOE4 carriers, the BBB was intact in cognitively healthy APOE3 carriers. It was, however, leaky in APOE3 carriers who showed cognitive impairment — although less so than in APOE4 carriers at an equivalent stage of impairment.
Next, Montagne et al. examined whether BBB breakdown in APOE4 carriers was linked to pericyte degeneration. In support of this idea, they found that a biomarker of pericyte injury — a soluble form of a protein known as platelet-derived growth factor-receptor-β (sPDGFRβ) — was elevated in the cerebrospinal fluid of APOE4 carriers compared with APOE3 carriers. High levels of the protein in people who carried APOE4 were associated with a leaky BBB and cognitive impairment. sPDGFRβ elevation was independent of Aβ and tau.
The authors then looked for insight into the mechanisms by which pericytes might become injured. They focused on cyclophilin A (CypA) and matrix metalloproteinase-9 (MMP9), two proteins that are part of an inflammatory pathway implicated in APOE4-driven pericyte damage and BBB breakdown6. Levels of CypA and MMP9 in the cerebrospinal fluid were higher in APOE4 carriers who had mild cognitive impairment than in cognitively healthy APOE4 carriers or APOE3 carriers who had comparable cognitive dysfunction. Again, this change was not related to increases in Aβ or tau.
Finally, the researchers generated pericytes in vitro from human induced pluripotent stem cells that expressed APOE3 or APOE4. They found that APOE4-expressing pericytes secreted substantially more CypA and MMP9 than did APOE3 pericytes. ApoE4 (but not ApoE3) secreted by pericytes activates the CypA–MMP9 pathway on nearby pericytes — the cells therefore cause their own demise. ApoE4 could also activate the CypA–MMP9 pathway in endothelial cells, which are susceptible to the harmful effects of APOE47. Therefore, injury to pericytes and endothelial cells might both cause BBB leakage (Fig. 1).

Figure 1 | The gene variant *APOE4* and Alzheimer’s disease. People who carry APOE4 are at heightened risk of Alzheimer’s disease. Montagne et al.3 provide evidence that ApoE4 protein is secreted by cells called pericytes, which abut endothelial cells that line cerebral capillaries at the blood–brain barrier (BBB). Secreted ApoE4 activates the protein cyclophilin A (CypA) in the pericytes. This triggers a downstream signalling pathway involving activation of the inflammatory protein matrix metalloproteinase-9 (MMP9) in pericytes, and possibly also in endothelial cells. This causes disruption of junctions between adjoining endothelial cells, opening the BBB in brain regions involved in learning and memory. Disruption of the BBB is associated with impaired cognition, although the mechanisms that link the two are unclear.
See you tomorrow